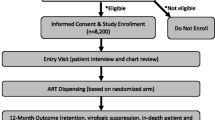
Abstract
HIV remission trials often require temporary stopping of antiretroviral therapy (ART)—an approach called analytic treatment interruption (ATI). Trial designs resulting in viremia raise risks for participants and sexual partners. We conducted a survey on attitudes about remission trials, comparing ART resumption criteria (lower-risk “time to rebound” and higher-risk “sustained viremia”) among participants from an acute HIV cohort in Thailand. Analyses included Wilcoxon-Ranks and multivariate logistic analysis. Most of 408 respondents supported ATI trials, with slightly higher approval of, and willingness to participate in, trials using time to rebound versus sustained viremia criteria. Less than half of respondents anticipated disclosing trial participation to partners and over half indicated uncertainty or unwillingness about whether partners would be willing to use PrEP. Willingness to participate was higher among those who rated higher trial approval, lower anticipated burden, and those expecting to make the decision independently. Our findings support acceptability of ATI trials among most respondents. Participant attitudes and anticipated behaviors, especially related to transmission risk, have implications for future trial design and informed consent.

Similar content being viewed by others
Data Availability
De-identified data are available upon request.
Code Availability
Code is available upon request.
References
Wen Y, Bar KJ, Li JZ. Lessons learned from HIV antiretroviral treatment interruption trials. Curr Opin HIV AIDS. 2018. https://doi.org/10.1097/COH.0000000000000484.
Eyal N, Holtzman LG, Deeks SG. Ethical issues in HIV remission trials. Curr Opin HIV AIDS. 2018;13(5):422–7.
Muccini C, Crowell TA, Kroon E, Sacdalan C, Ramautarsing R, Seekaew P, et al. Leveraging early HIV diagnosis and treatment in Thailand to conduct HIV cure research. AIDS Res Ther. 2019. https://doi.org/10.1186/s12981-019-0240-4.
Henderson GE, Peay HL, Kroon E, Cadigan RJ, Meagher K, Jupimai T, et al. Ethics of treatment interruption trials in HIV cure research: addressing the conundrum of risk/benefit assessment. J Med Ethics. 2018;44(4):270–6.
Henderson GE, Waltz M, Meagher K, Cadigan RJ, Jupimai T, Isaacson S, et al. Going off antiretroviral treatment in a closely monitored HIV “cure” trial: longitudinal assessments of acutely diagnosed trial participants and decliners. J Int AIDS Soc. 2019;22(3):e25260.
Crowell TA, Colby DJ, Pinyakorn S, Sacdalan C, Pagliuzza A, Intasan J, et al. Safety and efficacy of VRC01 broadly neutralising antibodies in adults with acutely treated HIV (RV397): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet HIV. 2019;6(5):e297–306.
Stecher M, Claßen A, Klein F, Lehmann C, Gruell H, Platten M, et al. Systematic review and meta-analysis of treatment interruptions in human immunodeficiency virus (HIV) type 1–infected patients receiving antiretroviral therapy: implications for future HIV cure trials. Clin Infect Dis. 2019;70(7):1406–17. https://doi.org/10.1093/cid/ciz417.
Borducchi EN, Cabral C, Stephenson KE, Liu J, Abbink P, Ng’ang’a D, et al. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature. 2016;540(7632):284–7.
Sneller MC, Justement JS, Gittens KR, Petrone ME, Clarridge KE, Proschan MA, et al. A randomized controlled safety/efficacy trial of therapeutic vaccination in HIV-infected individuals who initiated antiretroviral therapy early in infection. Sci Transl Med. 2017. https://doi.org/10.1126/scitranslmed.aan8848.
Julg B, Dee L, Ananworanich J, Barouch DH, Bar K, Caskey M, et al. Recommendations for analytical antiretroviral treatment interruptions in HIV research trials—report of a consensus meeting. Lancet HIV. 2019. https://doi.org/10.1016/S2352-3018(19)30052-9.
Jefferys R. Community recommendations for clinical research involving antiretroviral treatment interruption in adults. 2018. https://www.treatmentactiongroup.org/wp-content/uploads/2018/11/community_recs_clinical_research_final.pdf. Accessed 11 Oct 2021.
Palich R, Ghosn J, Chaillon A, Boilet V, Nere M-L, Chaix M-L, et al. Viral rebound in semen after antiretroviral treatment interruption in an HIV therapeutic vaccine double-blind trial. AIDS. 2019;33(2):279–84. https://doi.org/10.1097/QAD.0000000000002058.
Ugarte A, Romero Y, Tricas A, Casado C, Lopez-Galindez C, Garcia F, et al. Unintended HIV-1 infection during analytical therapy interruption. J Infect Dis. 2020;221(10):1740–2. https://doi.org/10.1093/infdis/jiz611.
Lau JSY, Smith MZ, Allan B, Martinez C, Power J, Lewin SR, et al. Perspectives on analytical treatment interruptions in people living with HIV and their health care providers in the landscape of HIV cure-focused studies. AIDS Res Hum Retrovir. 2020. https://doi.org/10.1089/aid.2019.0118.
Eyal N, Deeks SG. Risk to nonparticipants in HIV remission studies with treatment interruption: a symposium. J Infect Dis. 2019;220(220 Suppl 1):S1–4.
Peluso MJ, Dee L, Campbell D, Taylor J, Hoh R, Rutishauser RL, et al. A collaborative, multidisciplinary approach to HIV transmission risk mitigation during analytic treatment interruption. J Virus Erad. 2020;6(1):34–7.
Evans D. An activist’s argument that participant values should guide risk-benefit ratio calculations in HIV cure research. J Med Ethics. 2017;43(2):100–3.
Peay HL, Henderson GE. What motivates participation in HIV cure trials? A call for real-time assessment to improve informed consent. J Virus Erad. 2015;1(2):51–3.
Dubé K, Evans D, Sylla L, Taylor J, Weiner BJ, Skinner A, et al. Willingness to participate and take risks in HIV cure research: survey results from 400 people living with HIV in the US. J Virus Erad. 2017. https://doi.org/10.1016/S2055-6640(20)30295-8.
Dubé K, Evans D, Dee L, Sylla L, Taylor J, Skinner A, et al. “We need to deploy them very thoughtfully and carefully”: perceptions of analytical treatment interruptions in HIV cure research in the United States-a qualitative inquiry. AIDS Res Hum Retrovir. 2018;34(1):67–79.
Protière C, Spire B, Mora M, Poizot-Martin I, Préau M, Doumergue M, et al. Patterns of patient and healthcare provider viewpoints regarding participation in HIV cure-related clinical trials. Findings from a multicentre French survey using Q methodology (ANRS-APSEC). PLoS ONE. 2017;12(11):e0187489–e0187489.
Protiere C, Arnold M, Fiorentino M, Fressard L, Lelièvre JD, Mimi M, et al. Differences in HIV cure clinical trial preferences of French people living with HIV and physicians in the ANRS-APSEC study: a discrete choice experiment. J Int AIDS Soc. 2020;23(2):e25443–e25443.
CDC. National health and nutrition examination survey. CDC. 2015. https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/HSQ_H.htm. Accessed 11 Oct 2021.
Broadbent E, Petrie K, Main J, Weinman J. The brief illness perception questionnaire (BIPQ). J Psychosom Res. 2006;1(60):631–7.
Peay, H.L., Jupimai, T., Ormsby, N., Rennie, S., Cadigan, R.J, Kuczynski, K., Isaacson, S.C., Phanuphak, N., Kroon, E., Ananworanich, J., Vasan, S., Prueksakaew, P., Intasan, J., Henderson GE. Perceived health and stigma among a population of individuals diagnosed with acute HIV: report from the SEARCH010 cohort. In: AIDS 2020: virtual [conference presentation]. San Francisco: International AIDS Society.
Knobel H, Alonso J, Casado JL, Collazos J, González J, Ruiz I, et al. Validation of a simplified medication adherence questionnaire in a large cohort of HIV-infected patients: the GEEMA Study. AIDS. 2002. https://doi.org/10.1097/00002030-200203080-00012.
Khawcharoenporn T, Chunloy K, Apisarnthanarak A. Uptake of HIV testing and counseling, risk perception and linkage to HIV care among Thai university students. BMC Public Health. 2016;16:556.
Golin CE, Earp JA, Grodensky CA, Patel SN, Suchindran C, Parikh M, et al. Longitudinal effects of SafeTalk, a motivational interviewing-based program to improve safer sex practices among people living with HIV/AIDS. AIDS Behav. 2012;16(5):1182–91.
Phanuphak N, Ramautarsing R, Chinbunchorn T, Janamnuaysook R, Pengnonyang S, Termvanich K, et al. Implementing a status-neutral approach to HIV in the Asia-pacific. Curr HIV/AIDS Rep. 2020;17(5):422–30. https://doi.org/10.1007/s11904-020-00516-z.
Eyal N, Magalhaes M. Is it ethical to isolate study participants to prevent HIV transmission during trials with ananalytical treatment interruption? J Infect Dis. 2019;220(220 Suppl 1):S19–21. https://doi.org/10.1093/infdis/jiz164.
Henderson GE, Rennie S, Corneli A, Peay HL. Cohorts as collections of bodies and communities of persons: insights from the SEARCH010/RV254 research cohort. Int Health. 2020;12(6):584–90.
Acknowledgements
We would like to thank our study participants.
Funding
The study was funded in entirety by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (1R01AI127024). The investigators acknowledge expert input supported by the University of North Carolina at Chapel Hill Center for AIDS Research (P30 AI50410). The participants were from the RV254/SEARCH 010, which is supported by cooperative agreements (WW81XWH-18-2-0040) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., the U.S. Department of Defense (DOD), an intramural grant from the Thai Red Cross AIDS Research Centre, and by the U.S. National Institute of Allergy and Infectious Diseases. Antiretroviral therapy for RV254/SEARCH 010 participants was supported by the Thai Government Pharmaceutical Organization, Gilead, Merck and ViiV Healthcare. The funding sources have no involvement in study design, data collection and analysis, or writing the report.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design or the acquisition, analysis or interpretation of the data. Conceptualization and methodology were led by HP and GH. Data collection was led by TJ with support by PP and KB, with supervision by EK and DC. Analysis were performed by AG and HP. The first draft of the manuscript was written by HP, TJ, NO and GH, and all authors reviewed and revised previous versions of the manuscript. All authors read and approved the manuscript version to be published. All authors agree to be accountable for the work and ensure any questions about the accuracy or integrity are investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
DC has received research grant support from Gilead Sciences. All other authors report no conflicts of interest or competing interests.
Ethical Approval
This study was approved by the IRB of the Faculty of Medicine, Chulalongkorn University.
Consent to Participate
All participants provided informed consent to participate in the study.
Consent for Publication
The study informed consent included a section indicating that de-identified study data would be reported.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclaimer The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army or the Department of Defense. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70–25.
Appendix 1 Remission trial vignettes utilized in RV254 (SEARCH010) survey to assess trial attitudes and willingness to participate
Appendix 1 Remission trial vignettes utilized in RV254 (SEARCH010) survey to assess trial attitudes and willingness to participate
ATI vignette 1 Now, imagine you are invited to a new SEARCH 010 remission trial. It would test whether a new kind of experimental drug boosts the immune system, and if it is safe. You would get the experimental drug and then stop taking ART. You would come to SEARCH weekly for monitoring. The first time your viral load increases above 1000 copies/mL, you would restart ART About the experimental drug: Safety: The experimental drug would probably be safe, but researchers wouldn’t know for sure Side effects: You may have mild side effects While off ART: Monitoring at SEARCH: You would visit SEARCH once a week for health exams and blood draws to monitor your health, viral load, and CD4 count Side effects: You would probably not develop HIV symptoms or drug resistance. Longer term side effects are unknown Transmission risk: Your risk of transmitting HIV to partners might increase Re-starting ART: Viral load: People restart ART when their viral load increases above the trial’s limit (1000 copies/mL). For most people, this would be about 2 months after stopping ART, although a few people may stay off ART longer Researchers expect that once people restart ART, their viral load should decrease and become undetectable Long term monitoring: The SEARCH team would continue to monitor your health once a month for a year | ATI vignette 2 Now imagine there is a different type of SEARCH remission trial. This one keeps you off ART longer, which is riskier. This extra time off ART would test whether your immune system can recognize and kill the virus. The SEARCH team would check how your viral load goes up and down over time Like the first trial, You would get the same new experimental drug and then stop ART You would come in for a weekly health exam and blood draw If you have serious side effects from stopping ART at any time, you would immediately restart ART Once you restart ART, your viral load should decrease, and you should become undetectable again Once you restart ART, the SEARCH team would continue to monitor your health once a month for 1 year Different from the first trial Your viral load would be allowed to go over 1000 copies/mL to see if your own immune system would start to fight the HIV virus on its own If your viral load stays above 1000 copies/mL for 4 weeks in a row, you would restart ART As long as your viral load does NOT go over 1000 copies/mL for 4 weeks in a row, you would continue to stay off ART and be monitored weekly You would be at much higher risk of transmitting HIV to your partner(s), so you would need to use condoms or abstain from sex |
Rights and permissions
About this article
Cite this article
Peay, H.L., Rennie, S., Cadigan, R.J. et al. Attitudes About Analytic Treatment Interruption (ATI) in HIV Remission Trials with Different Antiretroviral Therapy (ART) Resumption Criteria. AIDS Behav 26, 1504–1516 (2022). https://doi.org/10.1007/s10461-021-03504-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-021-03504-5